
|
|
|
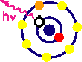
| X-ray Fluorescence is a process that occurs to relax an excited atom without a core electron. As shown in the figure below, an incident x-ray photon with sufficient energy can ionize a core electron. Naturally, any number of core electrons can be ionized from the numerous quantum electronic states that exist.
Once the absorptive process has completed, the atom then exists in an unstable configuration. It can relax through auger electron emission or through the process of fluorescence. In the Auger process, a higher energy electron decays to the state of the original core electron with the excess energy causing a less tightly bound electron to be emitted. For the fluorescence process, a decay from a higher electron shell emits an x-ray with corresponding energy difference. These processes are shown schematically below.
It should be noted that each of these decay mechanisms compete with each other, and that the process of fluorescence dominates for high Z elements, while auger electron emission is more probable for low Z elements.
|
Send mail to singhace@stanford.edu with
questions or comments about this web site.
|