Diamond 2.1: Adjusting bond spheres
Diamond 2.1 Features Overview...
Previous: Addressing of individual objects...
Next: Molecules and packing diagrams...
Diamond can create bonds without bonding informations in the input
file. The bonding spheres are calculated from mean bond length resources or
from effective radii.
Especially for metal-organic structures, the bonding spheres between metal and
C, O, N, etc., may be too short or too long. For that reason, Diamond
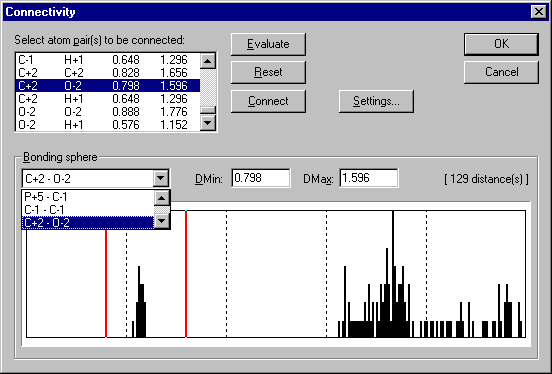
offers a very versatile histogram to adjust these spheres (the "connectivity"):
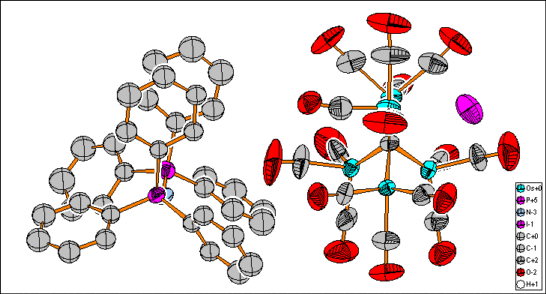
After checking the connectivity for example in Bis(triphenylphosphineiminium)
15-carbonylpentaosmium carbide iodide [1], the molecule creation function
generates a picture like this in one single step:
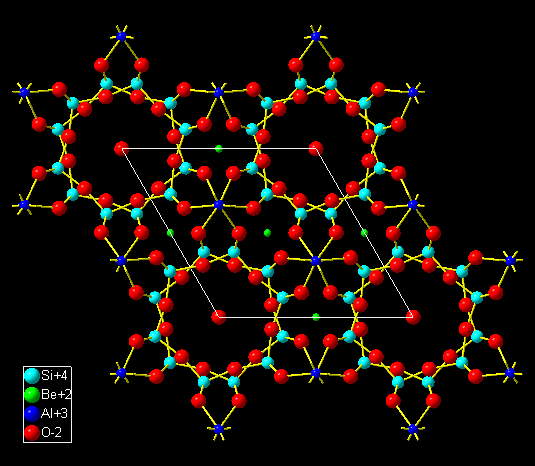
The selection of bonding spheres as well as the adjustment of the sphere sizes
can also be applied to inorganic structures that contain molecular subunits,
like in the mineral beryl [2]. After the import of the structural parameters,
Si-O, Be-O, and Al-O automatically have been pre-defined as connected.
Disabling bonds between Al and O as well as between Be and O leads to discrete
Si6O18 rings, whereas these units can be connected by
enabling the bonds between Al and O. Be and O are not connected in this
example:
References:
[1] Name: Bis(triphenylphosphineiminium) 15-carbonylpentaosmium carbide iodide
Formula: (((C6H5)3 P)2 N) (Os5
C(CO)15 I)
Author(s): Jackson P F, Johnson B F G, Lewis J, Nicholls J N, McPartlin M,
Nelson W J H
Title: Synthesis of the Carbido Anion (Os5 C (CO)15 I)
and the X-Ray Crystal Structures of Os5 C (CO)15 and ((Ph3
P)2 N) (Os5 C (CO)15 I)
Journal: JCCCA 1980 (1980), pp. 564 - 566
[2] ICSD Collection Code 2791
Name: Aluminium beryllium hexasilicate hydrate *
Formula: Al2 Be3 (SiO3)6 (H2O)0.0991
Author(s): Morosin, B
Title: Structure and thermal expansion of beryl
Journal: ACBCAR 28 (1972), pp. 1899 - 1903
Mineral name: Beryl
Diamond 2.1 Features Overview...
Previous: Addressing of individual objects...
Next: Molecules and packing diagrams...
|


