Diamond 2.1: Versatile use of polyhedra
Diamond 2.1 Features Overview...
Previous: Investigation of coordination spheres...
Next: ORTEP-like representation of thermal
ellipsoids...
Polyhedra are very useful to simplify the geometry of coordination spheres
around a real atom or a virtual center. For the display of polyhedra, Diamond
does not use a separate model (like e.g. wire or space-filling model) but uses
the default ("ball-and-stick") model. Thus polyhedra can be mixed with normal
atoms - as balls or as thermal ellipsoids - and bonds.
Diamond can construct polyhedra with up to 128 ligand atoms. Each polygon
(polyhedron face) can have up to eight corners (and must have at least three
corners, of course), but it must be convex. There is in principle no limit for
the maximum distance between central point and ligand atom. The size of the
enclosure sphere is either derived from the bonding sphere of the corresponding
atom types or has a discrete minimum and maximum value.
Polyhedron faces can be partially opened as well as being transparent, with or
without edges and with or without hatching. Colors for faces and edges can be
assigned globally or individually.
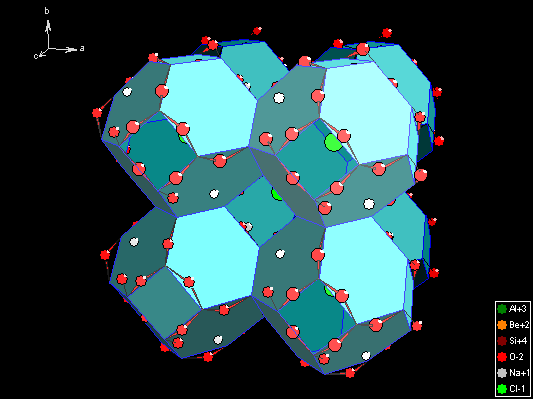
Example 1: Polyhedron framework of Sodalite [1] with rectangular and hexagonal
polygons and with partially open faces. The Diamond Tutorial (that is available
in both full and demonstration version) will show you how to build up such a
framework:
A polyhedron need not have a central atom. For this case, you may define a dummy
center, that means a position where is no real atom, or you may collect those
atoms that shall become the ligand atoms of the polyhedron.
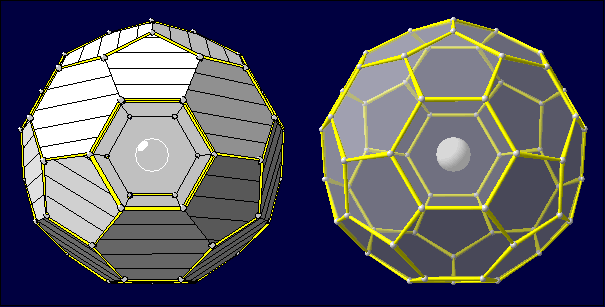
Example 2: Two representations of a fullerene molecule (C60) as
polyhedron with no real atom in its center and hexagonal and pentagonal faces.
The left version in flat representation with some faces open and the other
hatched, the right version using transparent faces (transparency factor: 0.5)
Reference:
[1] Name: Sodium tecto-alumoberyllotetrasilicate chloride
(8/2/2)
Formula: Na8 (Al Be Si4 O12)2 Cl2
Author(s): Hassan I, Grundy H D
Title: The crystal structure and thermal expansion of tugtupite,
Na8(Al2Be2Si8O24)Cl2
Journal: CAMIA 29 (1991), pp. 385 - 390
Mineral name: Tugtupite - from Ilimaussaq, South Greenland
Comment: TEM 1178
Diamond 2.1 Features Overview...
Previous: Investigation of coordination spheres...
Next: ORTEP-like representation of thermal
ellipsoids...
|


